Back
Present
Facts for Kids
Internal energy is the sum of the kinetic and potential energy of all particles in a system, influencing its temperature and phase.

Explore the internet with AstroSafe
Search safely, manage screen time, and remove ads and inappropriate content with the AstroSafe Browser.
Download
Inside this Article
Potential Energy
Thermal Energy
Thermodynamics
Temperature
Technology
Science
People
Matter
Energy
Did you know?
⚛️ Internal energy is the total energy contained within a system, including kinetic and potential energy of particles.
🌡️ It is a state function, meaning its value depends only on the current state of the system, not how it arrived there.
🧊 Internal energy increases as the temperature of a substance increases due to increased molecular motion.
🔋 In thermodynamics, internal energy is represented by the symbol 'U'.
🔄 Changes in internal energy are associated with heat transfer and work done on or by the system.
🧪 During a phase change, such as melting or boiling, internal energy changes while temperature remains constant.
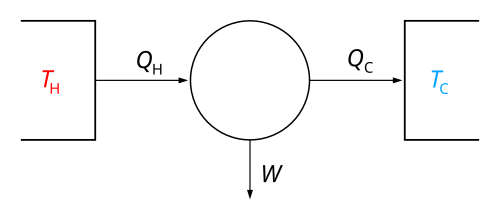
⚙️ The First Law of Thermodynamics states that the change in internal energy is equal to the heat added to the system minus the work done by the system.
🌌 Internal energy is fundamental to understanding processes in physics, chemistry, and engineering.
🌍 Different states of matter (solid, liquid, gas) have different internal energy due to differences in particle arrangement and movement.
🔍 When energy is added to a system, its internal energy increases, while removing energy will decrease it.
Show Less

Become a Creator with DIY.org
A safe online space featuring over 5,000 challenges to create, explore and learn in.
Learn more
Overview
Internal energy is a special kind of energy that's all around us! 🔋
It exists in every object, like our toys, the food we eat, and even in the air we breathe! This energy comes from tiny particles called atoms and molecules that make everything. When these particles move and wiggle faster, internal energy increases. 🌡
️ Scientists study internal energy to understand things like heat, temperature, and how energy changes during chemical reactions. Learning about internal energy helps us see how many things around us work, from cooking food to driving cars! 🚗
It exists in every object, like our toys, the food we eat, and even in the air we breathe! This energy comes from tiny particles called atoms and molecules that make everything. When these particles move and wiggle faster, internal energy increases. 🌡
️ Scientists study internal energy to understand things like heat, temperature, and how energy changes during chemical reactions. Learning about internal energy helps us see how many things around us work, from cooking food to driving cars! 🚗
Read Less
Forms of Internal Energy
There are different forms of internal energy! ⚛
️ One main type is thermal energy, which is all about heat. When you heat water for tea, the water molecules start moving faster, creating steam! 🌬
️ Another form is chemical energy, which is energy stored in food and fuels. When we eat, our bodies break food down to use that energy! 🍎
Lastly, there’s nuclear energy found in the center of atoms. This form of energy is super powerful, but we handle it carefully in nuclear plants. All these forms show that internal energy is important in our daily lives!
️ One main type is thermal energy, which is all about heat. When you heat water for tea, the water molecules start moving faster, creating steam! 🌬
️ Another form is chemical energy, which is energy stored in food and fuels. When we eat, our bodies break food down to use that energy! 🍎
Lastly, there’s nuclear energy found in the center of atoms. This form of energy is super powerful, but we handle it carefully in nuclear plants. All these forms show that internal energy is important in our daily lives!
Read Less
What is Internal Energy?
Internal energy is the total energy inside an object due to the motion and positions of its particles. Just like when you shake a bottle of soda, the fizz (gas) inside moves faster and creates bubbles! 🌌
In scientific terms, internal energy is made up of two parts: kinetic energy (energy of movement) and potential energy (stored energy). When these tiny particles in an object move quickly, like in hot soup 🍲, it has high internal energy. But when they are slow, like in ice, it has low internal energy. Joseph Thomson was an important scientist who discovered electrons and helped us understand internal energy. 🔍
In scientific terms, internal energy is made up of two parts: kinetic energy (energy of movement) and potential energy (stored energy). When these tiny particles in an object move quickly, like in hot soup 🍲, it has high internal energy. But when they are slow, like in ice, it has low internal energy. Joseph Thomson was an important scientist who discovered electrons and helped us understand internal energy. 🔍
Read Less
Measuring Internal Energy
Scientists measure internal energy using a special tool called a calorimeter! 📏
This tool helps them see how much heat energy is lost or gained during a process. To use it, scientists put the substance inside the calorimeter, heat it up, or add something cold, and then watch how the temperature changes. 🥵❄️ The change in temperature tells them about the internal energy! They also use the unit Joules (J) to measure energy, just like we use kilometers (km) for distance! Understanding how to measure internal energy helps scientists and engineers create better technology and understand nature! 🧪
This tool helps them see how much heat energy is lost or gained during a process. To use it, scientists put the substance inside the calorimeter, heat it up, or add something cold, and then watch how the temperature changes. 🥵❄️ The change in temperature tells them about the internal energy! They also use the unit Joules (J) to measure energy, just like we use kilometers (km) for distance! Understanding how to measure internal energy helps scientists and engineers create better technology and understand nature! 🧪
Read Less
Applications of Internal Energy
Internal energy is everywhere and used in many ways! 🌟
One cool application is in heating systems, like radiators in houses. They transform energy to keep us warm during winter ❄️. Another example is in refrigerators, which lower internal energy in food to keep it fresh! 🥦
Cars also use internal energy. They burn fuel to create energy, making parts move! 🚗
In science, researchers use internal energy to study weather patterns or even in space! Understanding internal energy helps us innovate and make the world a better place! 🌍✨
One cool application is in heating systems, like radiators in houses. They transform energy to keep us warm during winter ❄️. Another example is in refrigerators, which lower internal energy in food to keep it fresh! 🥦
Cars also use internal energy. They burn fuel to create energy, making parts move! 🚗
In science, researchers use internal energy to study weather patterns or even in space! Understanding internal energy helps us innovate and make the world a better place! 🌍✨
Read Less
The First Law of Thermodynamics
The First Law of Thermodynamics is like a magic rule for energy! ✨
It says energy can't be created or destroyed; it can only change from one form to another. For example, when you eat an apple 🍏, your body transforms the chemical energy in the apple into kinetic energy so you can run and play! 🏃
♂️ So if energy seems to disappear, it’s really just changing into another type. This law helps scientists understand how energy moves in different systems, like engines or even in nature, and it's super important in studying internal energy! 🌍
It says energy can't be created or destroyed; it can only change from one form to another. For example, when you eat an apple 🍏, your body transforms the chemical energy in the apple into kinetic energy so you can run and play! 🏃
♂️ So if energy seems to disappear, it’s really just changing into another type. This law helps scientists understand how energy moves in different systems, like engines or even in nature, and it's super important in studying internal energy! 🌍
Read Less
Factors Affecting Internal Energy
Several factors affect internal energy! 🌡
️ First, temperature plays a big role. When you heat something up, its particles move faster, increasing internal energy! 🥵
For example, if you put ice in a sunny spot, it will melt because the sun's heat makes the ice's internal energy rise. Another factor is the amount of substance. Adding more material, like water, increases internal energy because there are more particles to move! 🚰
Lastly, states of matter—solid, liquid, or gas—affect how the particles move and interact, changing the internal energy of matter!
️ First, temperature plays a big role. When you heat something up, its particles move faster, increasing internal energy! 🥵
For example, if you put ice in a sunny spot, it will melt because the sun's heat makes the ice's internal energy rise. Another factor is the amount of substance. Adding more material, like water, increases internal energy because there are more particles to move! 🚰
Lastly, states of matter—solid, liquid, or gas—affect how the particles move and interact, changing the internal energy of matter!
Read Less
Internal Energy in Chemical Reactions
Internal energy is really important in chemical reactions! 🔥
When you mix two substances, they sometimes react and change into new ones. This reaction can either release energy, making things warmer, or absorb energy, making things cooler. For example, when you light a match, the chemical energy in the matchstick turns into heat and light energy! 💥
This is why fireworks burst with colors and sounds! Other reactions, like baking cookies 🍪, use the heat from your oven to change dough into delicious treats! Understanding internal energy helps us discover how reactions happen and why they are important in our lives!
When you mix two substances, they sometimes react and change into new ones. This reaction can either release energy, making things warmer, or absorb energy, making things cooler. For example, when you light a match, the chemical energy in the matchstick turns into heat and light energy! 💥
This is why fireworks burst with colors and sounds! Other reactions, like baking cookies 🍪, use the heat from your oven to change dough into delicious treats! Understanding internal energy helps us discover how reactions happen and why they are important in our lives!
Read Less
Common Misconceptions about Internal Energy
Many people get confused about internal energy! One common misconception is thinking that it's just heat. 🔥
While heat is one part, internal energy includes kinetic and potential energy too! Another misunderstanding is that objects must be warm to have internal energy. That's not true! ❄
️ Even ice has internal energy; it just moves slowly! Some think energy can be created or destroyed, but remember the First Law of Thermodynamics says it can only change forms! By learning about the real facts of internal energy, we can understand the world better! 🌈
While heat is one part, internal energy includes kinetic and potential energy too! Another misunderstanding is that objects must be warm to have internal energy. That's not true! ❄
️ Even ice has internal energy; it just moves slowly! Some think energy can be created or destroyed, but remember the First Law of Thermodynamics says it can only change forms! By learning about the real facts of internal energy, we can understand the world better! 🌈
Read Less
Try your luck with the Internal Energy Quiz.
Try this Internal Energy quiz and see how many you score!
Q1
Question 1 of 10
Next
Explore More