Back
Present
Facts for Kids
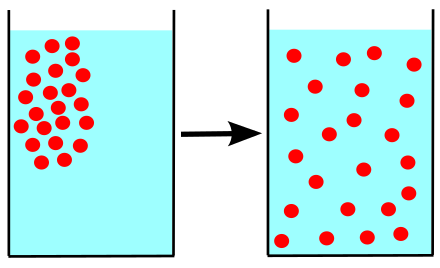
Diffusion is the process of particles moving from a place where they are many to a place where they are few.

Explore the internet with AstroSafe
Search safely, manage screen time, and remove ads and inappropriate content with the AstroSafe Browser.
Download
Inside this Article
Carbon Dioxide
Concentration
Medicine
Perfume
Science
Osmosis
Oxygen
Factor
Pebble
Glass
Did you know?
🌍 Diffusion is the movement of particles from areas of high concentration to low concentration.
🌊 Osmosis is a special type of diffusion that specifically involves water.
⚡️ Higher temperatures speed up diffusion because particles move faster.
🍕 Scents from food can diffuse in the air, making us feel hungry!
🩸 Our bodies use diffusion to bring oxygen into our blood.
🌿 Plants rely on diffusion to take in carbon dioxide for photosynthesis.
🧐 Fick's Laws of Diffusion tell us how fast particles diffuse based on concentration differences.
⏱️ Scientists can measure diffusion by timing how fast a dye spreads in water.
🌈 Diffusion occurs in gases, liquids, and solids but at different rates.
💧 If you put a raisin in water, it swells due to osmosis, a type of diffusion!
Show Less

Become a Creator with DIY.org
A safe online space featuring over 5,000 challenges to create, explore and learn in.
Learn more
Overview
Diffusion is a cool process that happens all around us! 🌍
It is when things, like smells or colors, move from a place where there are many of them (high concentration) to a place where there are not many (low concentration). Imagine you're in a room where someone sprays perfume. At first, the smell is very strong near the spray, but soon, you can smell it all over the room! This works because of diffusion. It helps mix things up and is super important for life!
It is when things, like smells or colors, move from a place where there are many of them (high concentration) to a place where there are not many (low concentration). Imagine you're in a room where someone sprays perfume. At first, the smell is very strong near the spray, but soon, you can smell it all over the room! This works because of diffusion. It helps mix things up and is super important for life!
Read Less
Types of Diffusion
There are three main types of diffusion! The first is simple diffusion, where small particles move through a barrier, like oxygen moving into our blood. The second is facilitated diffusion, which helps larger particles through a special gate in a cell's wall. Finally, we have osmosis, which is specifically the diffusion of water across a barrier. 🌊
Each type plays a unique role in moving things in nature and our bodies!
Each type plays a unique role in moving things in nature and our bodies!
Read Less
Diffusion vs. Osmosis
Diffusion and osmosis are similar, but they're not the same! 🤔
While diffusion is the movement of all kinds of particles, osmosis specifically deals with water. In osmosis, water moves through a semi-permeable membrane – that’s a fancy term for a barrier! For example, if you put a raisin in water, water diffuses into the raisin, making it swell and become plump again! 🍇
So, osmosis is like a special type of diffusion for water!
While diffusion is the movement of all kinds of particles, osmosis specifically deals with water. In osmosis, water moves through a semi-permeable membrane – that’s a fancy term for a barrier! For example, if you put a raisin in water, water diffuses into the raisin, making it swell and become plump again! 🍇
So, osmosis is like a special type of diffusion for water!
Read Less
Fick's Laws of Diffusion
Fick's Laws help scientists understand how diffusion happens. 📏
The first law says that when there's a big difference in concentration, diffusion happens quickly. The second law tells us how the concentration changes over time. These laws are super helpful, especially in science experiments and studies! They help scientists create medical treatments and understand how pollutants spread in the air or water! 🌫
️
The first law says that when there's a big difference in concentration, diffusion happens quickly. The second law tells us how the concentration changes over time. These laws are super helpful, especially in science experiments and studies! They help scientists create medical treatments and understand how pollutants spread in the air or water! 🌫
️
Read Less
Applications of Diffusion
Diffusion has many real-world uses! For example, scents from food can spread through the air in a kitchen, making everyone hungry! 🍕
In medicine, diffusing medicines into the bloodstream helps people feel better. Scientists use diffusion to understand how substances move in nature, like in oceans or even in the air. It also helps us create delicious drinks, like when flavors mix in lemonade! 🍋
In medicine, diffusing medicines into the bloodstream helps people feel better. Scientists use diffusion to understand how substances move in nature, like in oceans or even in the air. It also helps us create delicious drinks, like when flavors mix in lemonade! 🍋
Read Less
Factors Affecting Diffusion
Several factors affect how fast diffusion happens! ⚡
️ One big factor is the concentration. If there are a lot of particles packed together, they diffuse faster to less crowded places. Temperature also matters; warmer temperatures make particles move quicker. Lastly, the size of particles counts! Smaller particles can squeeze through spaces more easily than larger ones. Together, these factors help control how diffusion works in different situations.
️ One big factor is the concentration. If there are a lot of particles packed together, they diffuse faster to less crowded places. Temperature also matters; warmer temperatures make particles move quicker. Lastly, the size of particles counts! Smaller particles can squeeze through spaces more easily than larger ones. Together, these factors help control how diffusion works in different situations.
Read Less
Measurement of Diffusion Rates
Scientists measure how fast diffusion occurs using different methods! ⏱
️ One way is to look at how long it takes for a dye to spread in water. For example, if you drop food coloring into a glass of water, they can time how quickly the color spreads! The faster it spreads, the higher the diffusion rate. Scientists can compare diffusion rates in different environments, like warm vs. cold water or small vs. large openings. This helps them learn more about the world!
️ One way is to look at how long it takes for a dye to spread in water. For example, if you drop food coloring into a glass of water, they can time how quickly the color spreads! The faster it spreads, the higher the diffusion rate. Scientists can compare diffusion rates in different environments, like warm vs. cold water or small vs. large openings. This helps them learn more about the world!
Read Less
Diffusion in Biological Systems
In our bodies, diffusion is super important! 🩸
For example, oxygen from the air we breathe diffuses into our blood because there are fewer oxygen molecules in our blood than in the air. Similarly, waste products from our cells need to diffuse out into the blood so we can get rid of them. Plants also use diffusion to take in carbon dioxide from the air for photosynthesis, helping them grow and produce food. 🌿
For example, oxygen from the air we breathe diffuses into our blood because there are fewer oxygen molecules in our blood than in the air. Similarly, waste products from our cells need to diffuse out into the blood so we can get rid of them. Plants also use diffusion to take in carbon dioxide from the air for photosynthesis, helping them grow and produce food. 🌿
Read Less
Real-World Examples of Diffusion
Diffusion happens all around us every day! 🌈
For example, when you add sugar to your tea, the sugar moves from the spoon to mix evenly in the drink! Similarly, if you drop a pebble into a pond, the ripples spread out, showing how water and energy diffuse. In nature, when flowers bloom, their scent diffuses to attract bees and butterflies! 🐝
Each example helps us understand how important diffusion is in our daily lives!
For example, when you add sugar to your tea, the sugar moves from the spoon to mix evenly in the drink! Similarly, if you drop a pebble into a pond, the ripples spread out, showing how water and energy diffuse. In nature, when flowers bloom, their scent diffuses to attract bees and butterflies! 🐝
Each example helps us understand how important diffusion is in our daily lives!
Read Less
Diffusion in Gases, Liquids, and Solids
Diffusion doesn’t just happen in one state of matter! 🌟
It occurs in gases, liquids, and solids, but it’s different in each. In gases, particles move quickly and spread over a large space, like how perfume fills a room. In liquids, like water, diffusion is slower because particles are closer together. In solids, diffusion happens, but it's very slow since particles are tightly packed, like salt dissolving in ice! Each state has its own special diffusion properties!
It occurs in gases, liquids, and solids, but it’s different in each. In gases, particles move quickly and spread over a large space, like how perfume fills a room. In liquids, like water, diffusion is slower because particles are closer together. In solids, diffusion happens, but it's very slow since particles are tightly packed, like salt dissolving in ice! Each state has its own special diffusion properties!
Read Less
Try your luck with the Diffusion Quiz.
Try this Diffusion quiz and see how many you score!
Q1
Question 1 of 10
Next
Explore More