Back
Present
Facts for Kids
A chemical element is a special kind of building block made of atoms, all containing the same number of protons, that creates everything around us.

Explore the internet with AstroSafe
Search safely, manage screen time, and remove ads and inappropriate content with the AstroSafe Browser.
Download

Become a Creator with DIY.org
A safe online space featuring over 5,000 challenges to create, explore and learn in.
Learn more
Overview
A chemical element is a special kind of building block that makes up everything around us! 🌍
Each element is made of tiny particles called atoms, which all have the same number of protons (these are positively charged particles). For example, hydrogen is the simplest element with just ONE proton! There are 118 known elements on the Periodic Table, ranging from hydrogen (H) to oganesson (Og). Elements can be metals, non-metals, or metalloids, and they help create all the things we use in daily life, like water, air, and even the food we eat! 🍎
Each element is made of tiny particles called atoms, which all have the same number of protons (these are positively charged particles). For example, hydrogen is the simplest element with just ONE proton! There are 118 known elements on the Periodic Table, ranging from hydrogen (H) to oganesson (Og). Elements can be metals, non-metals, or metalloids, and they help create all the things we use in daily life, like water, air, and even the food we eat! 🍎
Read Less
Industrial Uses
Elements are essential in industries that help create everyday items! 🏭
For example, aluminum (Al) is strong yet lightweight, making it perfect for cans and airplanes! ⚙
️ Copper (Cu) is excellent for electrical wiring because it conducts electricity well. Even silicon (Si) is crucial in making computer chips! 💻
Elements also play a role in medicine, like using helium in MRI machines! The diverse uses of elements in various industries help improve our lives, from transportation to technology and healthcare! Understanding these uses shows how powerful chemistry can be in the world! 🌍
For example, aluminum (Al) is strong yet lightweight, making it perfect for cans and airplanes! ⚙
️ Copper (Cu) is excellent for electrical wiring because it conducts electricity well. Even silicon (Si) is crucial in making computer chips! 💻
Elements also play a role in medicine, like using helium in MRI machines! The diverse uses of elements in various industries help improve our lives, from transportation to technology and healthcare! Understanding these uses shows how powerful chemistry can be in the world! 🌍
Read Less
Atomic Structure
Atoms are like little LEGO blocks! 🧱
Each atom has three main parts: protons, neutrons, and electrons. Protons are in the center or nucleus of the atom and determine what kind of element it is. For example, if there are 6 protons, it's carbon (C)! Neutrons also sit in the nucleus and give the atom weight, but they don’t affect the type of element. Electrons are much smaller and zoom around the nucleus. They have a negative charge. Their arrangement helps atoms connect with each other to form different things, just like how connecting LEGO pieces makes new shapes! 🔗
Each atom has three main parts: protons, neutrons, and electrons. Protons are in the center or nucleus of the atom and determine what kind of element it is. For example, if there are 6 protons, it's carbon (C)! Neutrons also sit in the nucleus and give the atom weight, but they don’t affect the type of element. Electrons are much smaller and zoom around the nucleus. They have a negative charge. Their arrangement helps atoms connect with each other to form different things, just like how connecting LEGO pieces makes new shapes! 🔗
Read Less
Common Compounds
Compounds are what you get when elements join together! 🥳
For instance, when two hydrogen atoms bond with one oxygen atom, they create water (H₂O). Water is essential for life! Another common compound is carbon dioxide (CO₂), which is made of one carbon and two oxygen atoms. Plants need it to grow, using it during photosynthesis to make food and oxygen for us! 🌱
There are countless compounds, like vinegar (made of carbon, hydrogen, and oxygen) and table salt, each with unique properties and uses. Exploring compounds helps us understand our world better! 🌎
For instance, when two hydrogen atoms bond with one oxygen atom, they create water (H₂O). Water is essential for life! Another common compound is carbon dioxide (CO₂), which is made of one carbon and two oxygen atoms. Plants need it to grow, using it during photosynthesis to make food and oxygen for us! 🌱
There are countless compounds, like vinegar (made of carbon, hydrogen, and oxygen) and table salt, each with unique properties and uses. Exploring compounds helps us understand our world better! 🌎
Read Less
Interesting Facts
Did you know that helium (He) is the second most abundant element in the universe? 🌌
It’s lighter than air, which is why balloons filled with helium float! Another fun fact is that the element gallium (Ga) can melt in your hand because it has a low melting point! 🖐
️ Some elements glow, like phosphorus (P), which can shine in the dark after being exposed to light! Lastly, the most expensive element is californium (Cf), costing millions of dollars per gram! These fun facts show just how amazing and surprising the world of elements can be! 🌟
It’s lighter than air, which is why balloons filled with helium float! Another fun fact is that the element gallium (Ga) can melt in your hand because it has a low melting point! 🖐
️ Some elements glow, like phosphorus (P), which can shine in the dark after being exposed to light! Lastly, the most expensive element is californium (Cf), costing millions of dollars per gram! These fun facts show just how amazing and surprising the world of elements can be! 🌟
Read Less
Natural Occurrence
Elements can be found all around us in nature! 🌳
Some are abundant, while others are rare. For instance, oxygen (O) makes up about 21% of our atmosphere, allowing us to breathe! 🫁
On the other hand, gold (Au) is rare and valuable, often found in rivers or rocks. Many elements form minerals, like quartz (made of silicon and oxygen), which we can find in sand. Some elements, like iron (Fe), are often found in Earth's crust and are important for building things! Understanding where elements occur helps us find resources and use them wisely! 🔍
Some are abundant, while others are rare. For instance, oxygen (O) makes up about 21% of our atmosphere, allowing us to breathe! 🫁
On the other hand, gold (Au) is rare and valuable, often found in rivers or rocks. Many elements form minerals, like quartz (made of silicon and oxygen), which we can find in sand. Some elements, like iron (Fe), are often found in Earth's crust and are important for building things! Understanding where elements occur helps us find resources and use them wisely! 🔍
Read Less
Chemical Properties
Chemical properties tell us how elements act in different situations! 🧪
For example, sodium (Na) and chlorine (Cl) are both dangerous by themselves. But when they react together, they form table salt (NaCl), which we use every day to make our food tasty! 😋
Some elements love to make friends and bond with others, while some are loners. Metals tend to lose electrons easily, while non-metals gain or share electrons. Understanding chemical properties helps scientists predict how elements will react with each other, which is super important for making new materials and medicines! 🥼
For example, sodium (Na) and chlorine (Cl) are both dangerous by themselves. But when they react together, they form table salt (NaCl), which we use every day to make our food tasty! 😋
Some elements love to make friends and bond with others, while some are loners. Metals tend to lose electrons easily, while non-metals gain or share electrons. Understanding chemical properties helps scientists predict how elements will react with each other, which is super important for making new materials and medicines! 🥼
Read Less
Historical Discovery
The journey of discovering elements is fascinating! 🎉
The first known element, gold (Au), was used by ancient civilizations thousands of years ago! In the 1800s, scientist Dmitri Mendeleev organized the elements into the first Periodic Table, helping us understand them better! 📚
Other scientists, like Marie Curie, discovered radioactive elements like polonium and radium, unlocking secrets about atoms! Over time, scientists have discovered new elements, including the latest ones created in labs! This history of discovery shows how curious minds have shaped our understanding of the world around us! 🧠✨
The first known element, gold (Au), was used by ancient civilizations thousands of years ago! In the 1800s, scientist Dmitri Mendeleev organized the elements into the first Periodic Table, helping us understand them better! 📚
Other scientists, like Marie Curie, discovered radioactive elements like polonium and radium, unlocking secrets about atoms! Over time, scientists have discovered new elements, including the latest ones created in labs! This history of discovery shows how curious minds have shaped our understanding of the world around us! 🧠✨
Read Less
Isotopes and Stability
Did you know that some elements can have twins? 👫
These twins are called isotopes! Isotopes of an element have the same number of protons but a different number of neutrons. For example, carbon has two isotopes: carbon-12 with 6 neutrons and carbon-14 with 8 neutrons. Some isotopes are stable, meaning they don’t change over time. Others are radioactive, like carbon-14. This means they can break down and turn into different elements! Scientists use carbon-14 in dating ancient objects, like fossils, to learn about the past. ⏳
Isn’t science cool?
These twins are called isotopes! Isotopes of an element have the same number of protons but a different number of neutrons. For example, carbon has two isotopes: carbon-12 with 6 neutrons and carbon-14 with 8 neutrons. Some isotopes are stable, meaning they don’t change over time. Others are radioactive, like carbon-14. This means they can break down and turn into different elements! Scientists use carbon-14 in dating ancient objects, like fossils, to learn about the past. ⏳
Isn’t science cool?
Read Less
Reactivity and Bonding
Elements have different ways of making friends! 👫
Some elements, like hydrogen and oxygen, bond easily, while others, like noble gases (like helium and neon), prefer to stay alone. This is called reactivity! Elements react when they share, gain, or lose electrons to fill their outer shell. For example, when sodium meets chlorine, their electrons rearrange and form a strong bond, creating table salt. 🔗
Reactivity is influenced by how many electrons are in an element’s outer shell (valence electrons). Understanding these reactions helps scientists create new materials and medicines, making our lives better and more exciting! 🎉
Some elements, like hydrogen and oxygen, bond easily, while others, like noble gases (like helium and neon), prefer to stay alone. This is called reactivity! Elements react when they share, gain, or lose electrons to fill their outer shell. For example, when sodium meets chlorine, their electrons rearrange and form a strong bond, creating table salt. 🔗
Reactivity is influenced by how many electrons are in an element’s outer shell (valence electrons). Understanding these reactions helps scientists create new materials and medicines, making our lives better and more exciting! 🎉
Read Less
Periodic Table Placement
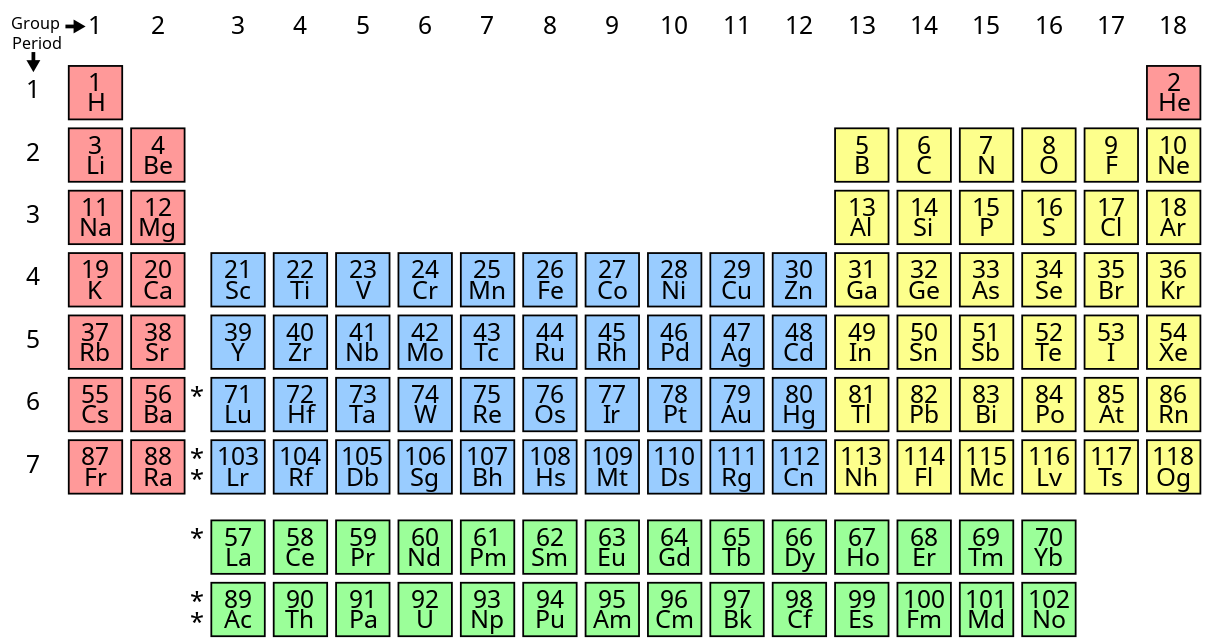
The Periodic Table is a special map that shows all the elements! 📊
Elements are arranged by their atomic number, which tells you how many protons are in each atom. For example, helium (He) has 2 protons, so it’s in the second spot! The table is divided into rows (periods) and columns (groups). Elements in the same group have similar properties. For instance, all the elements in group 1, like lithium (Li) and sodium (Na), are very reactive metals! Understanding where each element is placed helps scientists predict how they will behave in reactions! 🔍
Elements are arranged by their atomic number, which tells you how many protons are in each atom. For example, helium (He) has 2 protons, so it’s in the second spot! The table is divided into rows (periods) and columns (groups). Elements in the same group have similar properties. For instance, all the elements in group 1, like lithium (Li) and sodium (Na), are very reactive metals! Understanding where each element is placed helps scientists predict how they will behave in reactions! 🔍
Read Less
Try your luck with the Chemical Element Quiz.
Try this Chemical Element quiz and see how many you score!
Q1
Question 1 of 10
Next
Explore More